Файл:P450cycle.svg

Исходный файл (SVG-файл, номинально 9240 × 6968 пкс, размер файла: 38 КБ)
Описание
| Описание | ==The P450 catalytic cycle==
1: The substrate binds to the active site of the enzyme, in close proximity to the heme group, on the side opposite to the peptide chain. The bound substrate induces a change in the conformation of the active site, displacing a water molecule from the distal axial coordination position of the heme iron[#cite_note-P450Mechanism-1 [1]] changing the state of the heme iron from low-spin to high-spin[#cite_note-HiResCam-2 [2]]. This gives rise to a change in the spectral properties of the enzyme, with an increase in absorbance at 390~nm and a decrease at 420~nm. This can be measured by difference spectrometry and is referred to as the "type~I" difference spectrum (see inset graph in figure). Some substrates cause an opposite change in spectral properties, a "reverse type~I" spectrum, by processes that are as yet unclear. Inhibitors and certain substrates that bind directly to the heme iron give rise to the type~II difference spectrum, with a maximum at 430~nm and a minimum at 390~nm (see inset graph in figure). If no reducing equivalents are available, this complex remains stable, allowing the degree of binding to be determined from absorbance measurements in vitro[#cite_note-p450struc-3 [3]] 2: The change in the electronic state of the active site favours the transfer of an electron from NAD(P)H[#cite_note-P450pot-4 [4]]. This takes place via the electron transfer chain, as described above, reducing the ferric heme iron to the ferrous state. 3: Molecular oxygen binds covalently to the distal axial coordination position of the heme iron. The cysteine ligand is a better electron donor than histidine, with the oxygen consequently being activated to a greater extent than in other heme proteins. However, this sometimes allows the bond to dissociate, the so-called "decoupling reaction", releasing a reactive superoxide radical, interrupting the catalytic cycle[#cite_note-P450Mechanism-1 [1]]. 4: A second electron is transferred via the electron-transport system, reducing the dioxygen adduct to a negatively charged peroxo group. This is a short-lived intermediate state. 5: The peroxo group formed in step 4 is rapidly protonated twice by local transfer from surrounding amino-acid side chains, releasing one mole of water, and forming a highly reactive iron(V)-oxo species[#cite_note-P450Mechanism-1 [1]]. 6: Depending on the substrate and enzyme involved, P450 enzymes can catalyse any of a wide variety of reactions. A hypothetical hydroxylation is shown in this illustration. After the product has been released from the active site, the enzyme returns to its original state, with a water molecule returning to occupy the distal coordination position of the iron nucleus. S An alternative route for mono-oxygenation is via the "peroxide shunt": interaction with single-oxygen donors such as peroxides and hypochlorites can lead directly to the formation of the iron-oxo intermediate, allowing the catalytic cycle to be completed without going through steps 3, 4 and 5[#cite_note-p450struc-3 [3]]. A hypothetical peroxide "XOOH" is shown in the diagram. C: If carbon monoxide (CO) binds to reduced P450, the catalytic cycle is interrupted. This reaction yields the classic CO difference spectrum with a maximum at 450 nm.
|
|---|---|
| Источник | M.Sc. Thesis, David Richfield (User:Slashme) |
| Время создания | 2008-03 |
| Автор или правообладатель | When using this image in external works, it may be cited as follows:
|
| Другие версии файла | — |
Источник файла — сайт Wikimedia Commons, куда он был загружен под одной из свободных лицензий ( https://commons.wikimedia.org/wiki/File:P450cycle.svg ). Авторов, работавших над этим файлом см. в истории файла: https://commons.wikimedia.org/w/index.php?title=File:P450cycle.svg&action=history
В общем случае в статьях энциклопедии Руниверсалис файлы используются в соответствии со статьёй 1274 Гражданского кодекса Российской Федерации.
История файла
Нажмите на дату/время, чтобы увидеть версию файла от того времени.
| Дата/время | Миниатюра | Размеры | Участник | Примечание | |
|---|---|---|---|---|---|
| текущий | 15:11, 15 декабря 2023 | 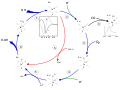 | 9240 × 6968 (38 КБ) | I, Robot (обсуждение | вклад) | == Описание == {{Изображение | описание = ==The P450 catalytic cycle== <p>1: The substrate binds to the active site of the enzyme, in close proximity to the heme group, on the side opposite to the peptide chain. The bound substrate induces a change in the conformation of the active site, displacing a water molecule from the distal axial coordination position of the heme iron<sup class="reference" id="cite_ref-P450Mechanism_1-0">[#cite_note-P450Mechanism-1 [1]]</sup> changing the state of th... |
Вы не можете перезаписать этот файл.
Использование файла
Следующий файл является дубликатом этого файла (подробности):
- Файл:P450cycle.svg из на Викискладе
Следующая страница использует этот файл: